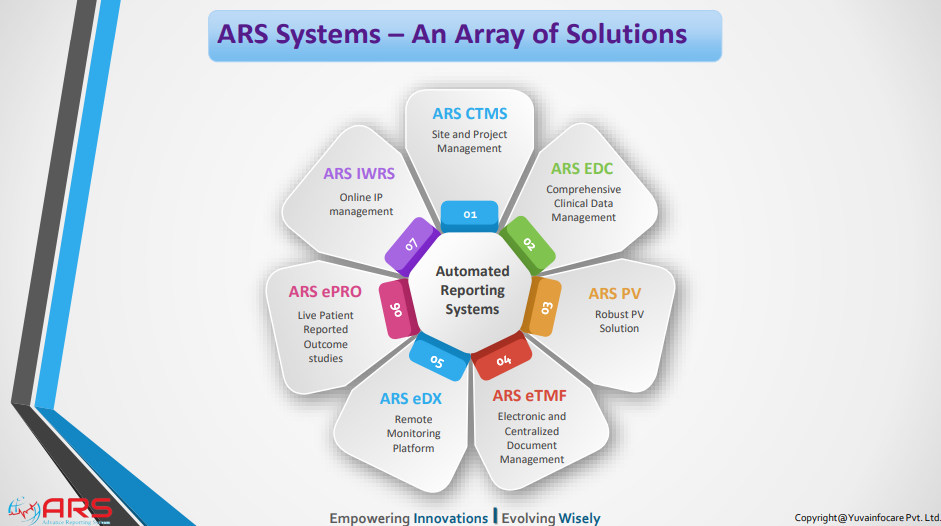
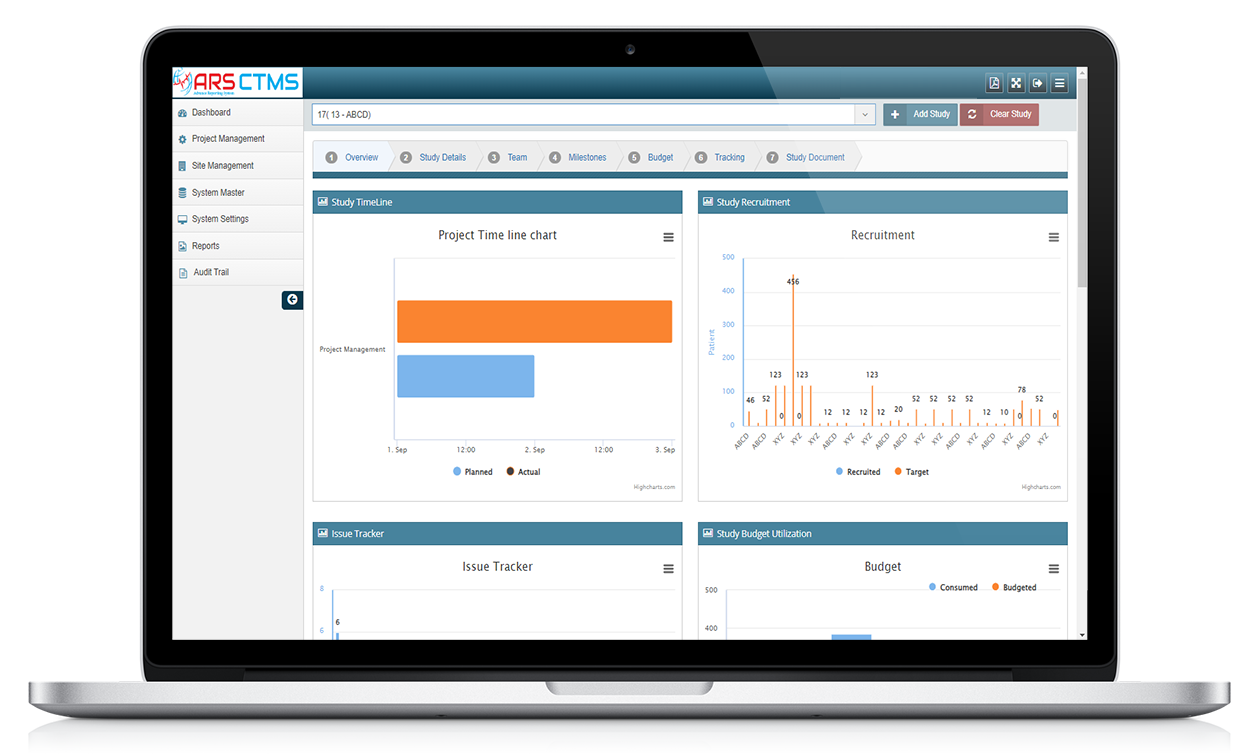
ARS CTMS
Protocol management

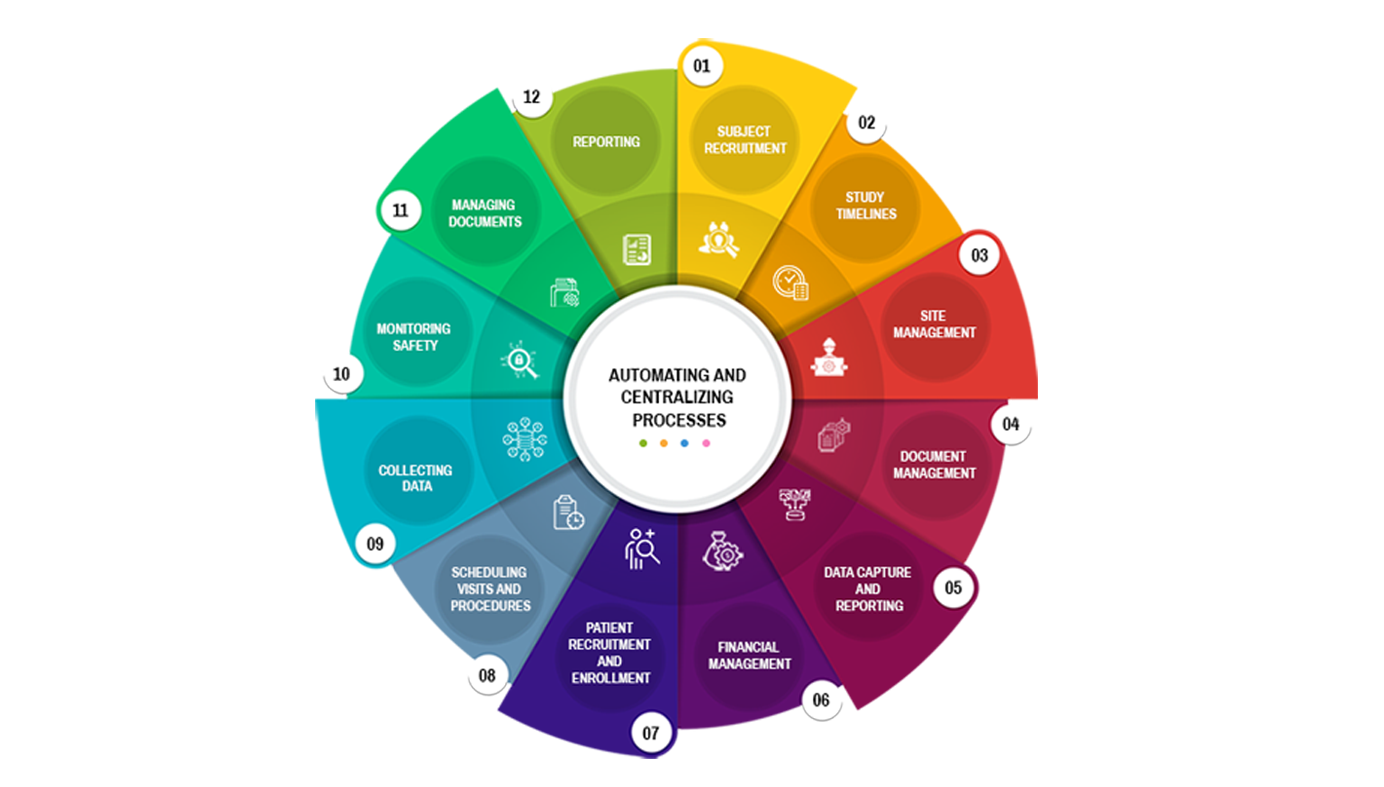
Subject recruitment and management
ARS CTMS can help manage the different study sites by providing tools to manage site initiation, site monitoring, and site closeout activities.
Site management
Document management
ARS CTMS can help manage the different documents associated with a clinical trial by providing tools to store, track, and share documents securely.
Data capture and reporting
Financial management
ARS CTMS can help manage the financial aspects of a clinical trial by providing tools to track expenses, payments, and budgets.
Compliance and regulatory management

Subject recruitment and management
ARS CTMS can help manage the recruitment of subjects by providing tools to screen, enrolment, and track the progress of study participants.
ARS PV
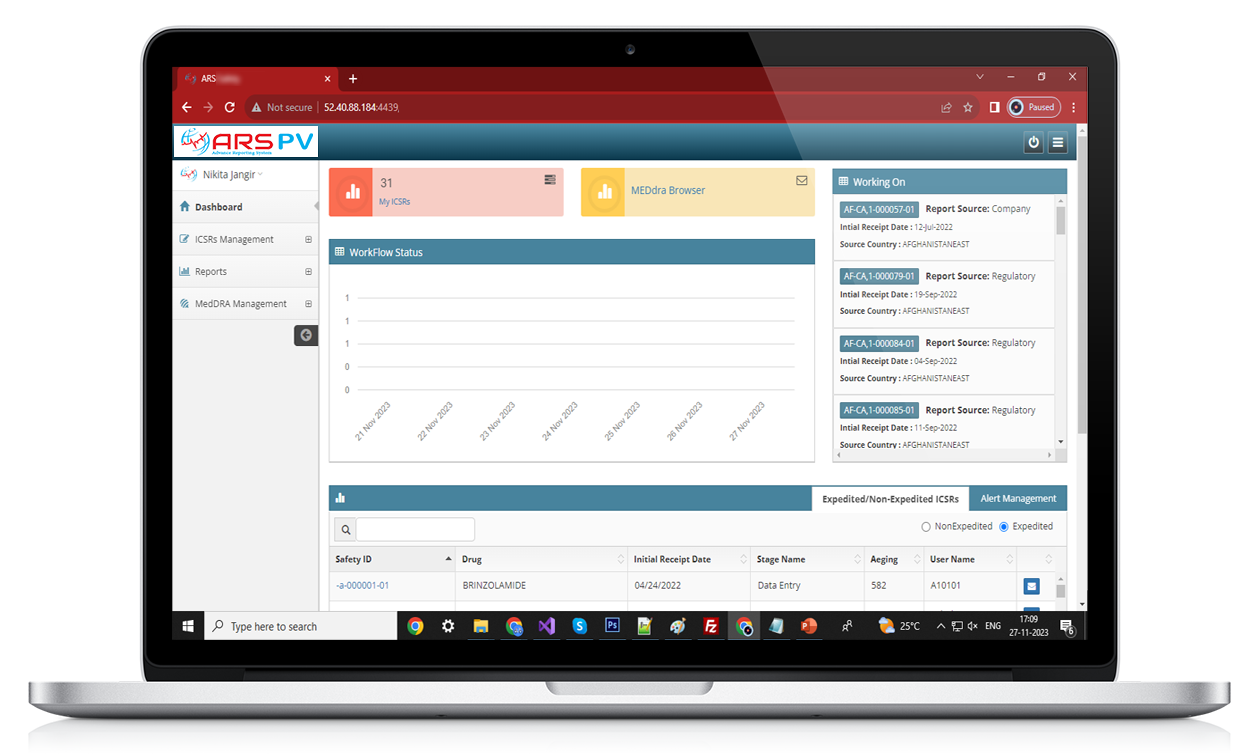
Features of ARS PV

▪ Managing multiple MedDRA version , its upgrade and impact analysis MedDRA version upgrade impact analysis
▪ Compliant with 21 CFR part 11 of US FDA ,GDPR, Annexure 11 of EMEA ,ICH GVP guidance like E2B R2 & R3 and GAMP 5 for computer system validation.
▪ Support Medical Drug dictionary and Medical Event Dictionary like (Drug Dictionary, WHO –Drug Dictionary and MedDRA).
▪ Enabling User with safety field specifications and user friendliness

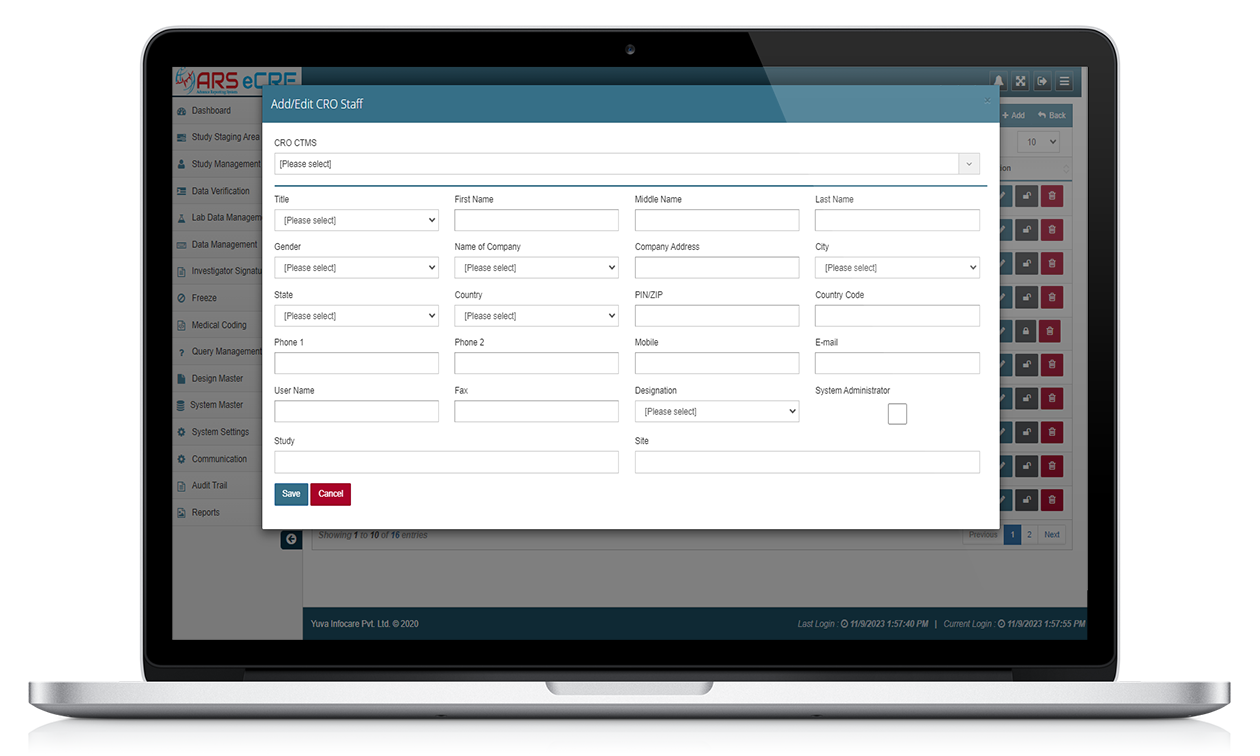
ARS EDC/ ARS eCRF
ARS EDC stands for Electronic Data Capture by Advance Reporting System powered by Yuva Infocare Pvt Ltd, which is a computerized system used in clinical trials to capture, manage, and report clinical trial data. ARS EDC replaces traditional paper-based data collection methods, making the process more efficient and accurate.
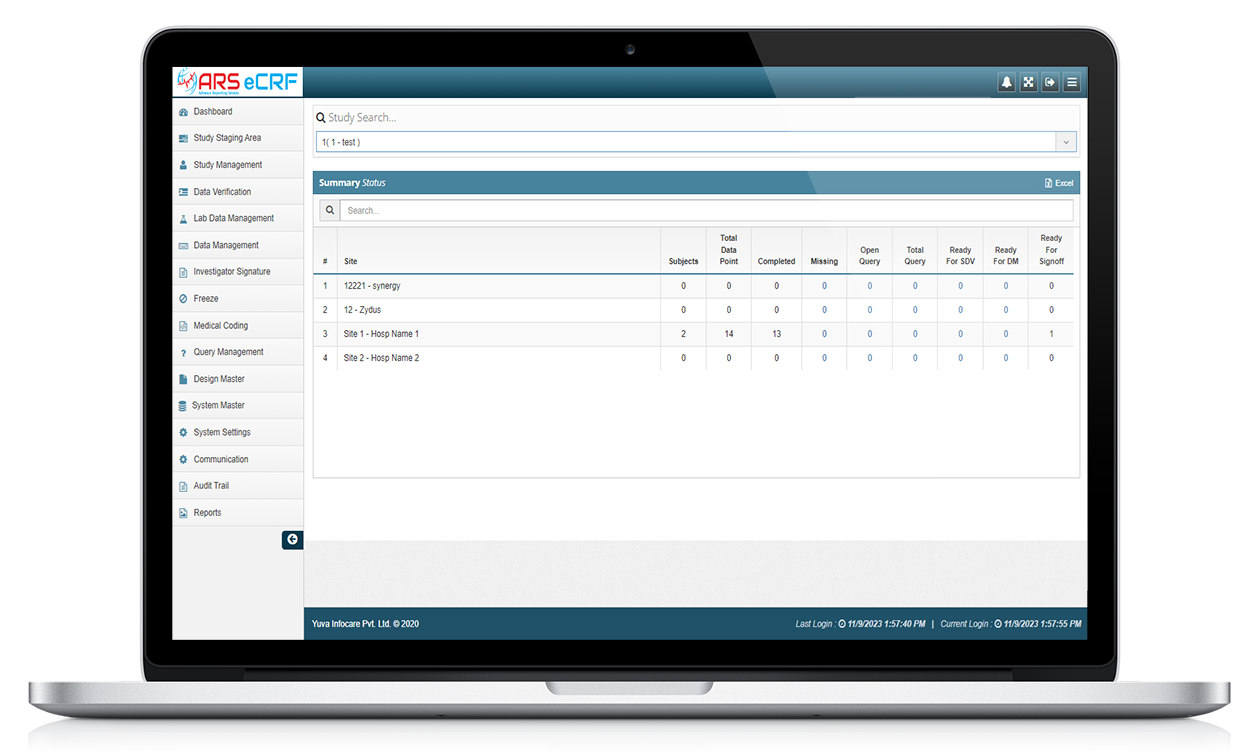
ARS EDC system allow for the electronic collection of clinical trial data, which can be entered directly into the system by the investigator or study coordinator, or through direct data capture from medical devices. ATS EDC system typically have features such as data validation and real-time data monitoring to ensure the accuracy and completeness of the data collected.
Some features of ARS EDC systems include
Data entry
ARS EDC systems provide electronic forms for entering data, replacing traditional paper-based forms.
Data monitoring
ARS EDC systems can monitor data entered into the system, alerting users to any data discrepancies or missing data. ARS EDC systems can provide real-time monitoring of study data, allowing for early identification of data anomalies or protocol violations. This can help reduce the risk of errors and ensure compliance with study protocols.
Remote monitoring
ARS EDC systems can enable remote monitoring of study data, allowing sponsors and monitors to access study data securely from anywhere in the world. This can help reduce the time and cost associated with on-site monitoring visits.
Data export
ARS EDC systems can export data in various formats, making it easy to share data with other researchers or regulatory agencies. This can facilitate the analysis and interpretation of study data.
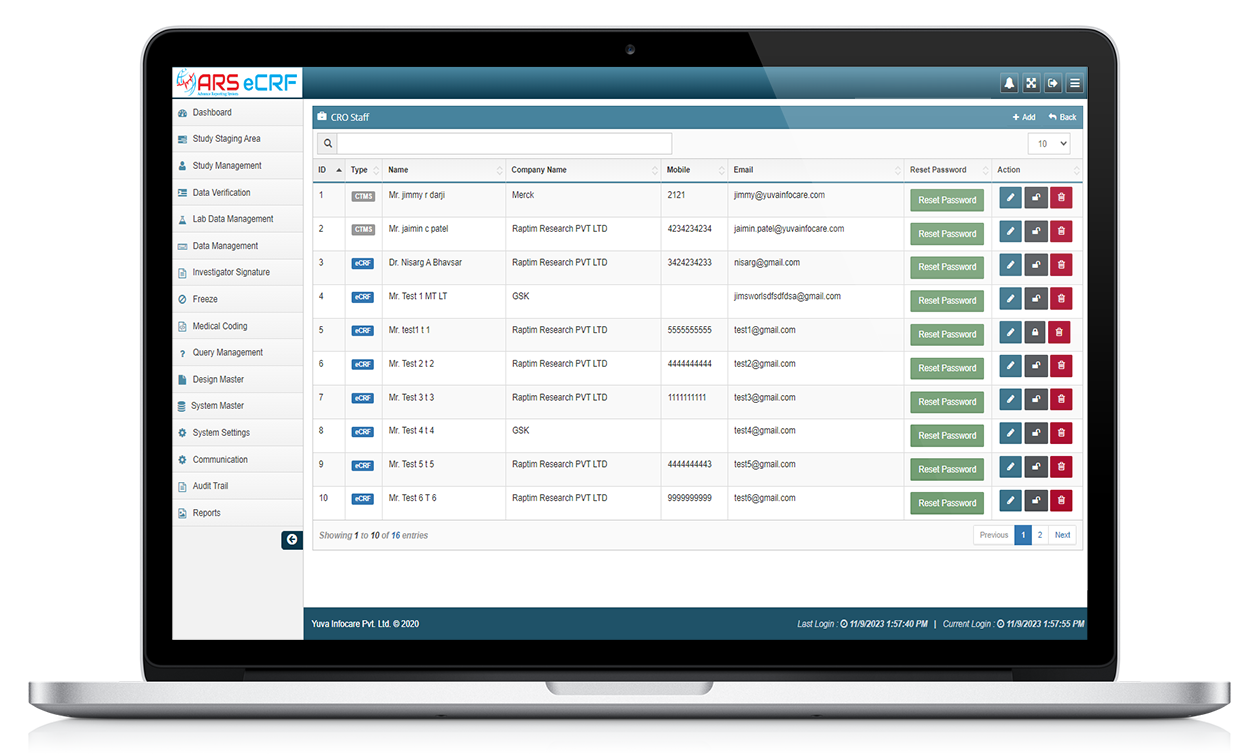
Overall, ARS EDC systems provide a more efficient and accurate way to collect and manage clinical trial data. They can improve data quality, reduce data entry errors, and provide real-time data monitoring, making them an essential tool for clinical trial management.
ARS EDC systems provide a powerful tool for the collection, management, and analysis of clinical trial data, improving efficiency, accuracy, and compliance in clinical research.
ARS IWRS
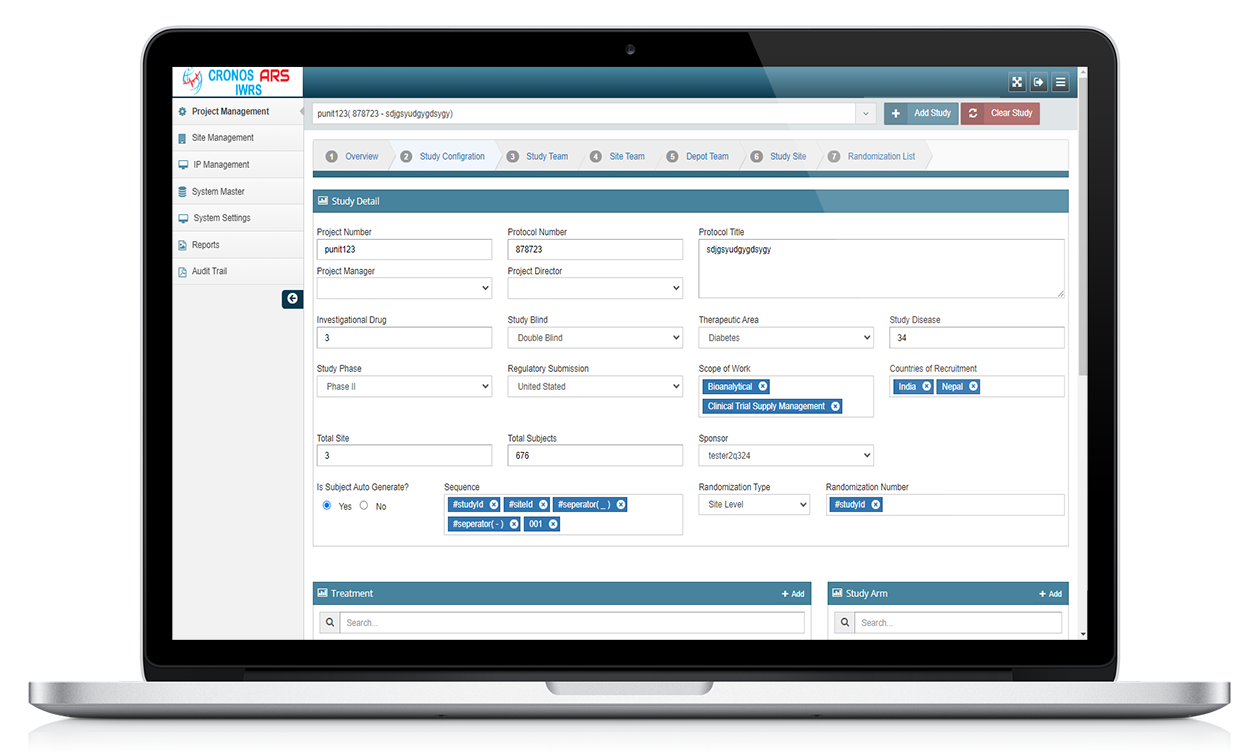
ARS IWRS stands for Interactive Web Response System powered by Yuva Infocare Pvt Ltd. It is a computerized system used in clinical trials and research studies to manage and control the randomization and drug supply processes.
The ARS IWRS system is typically accessed through a secure web portal and provides real-time interactions between the study site and the central study management team.
ARS IWRS system is typically used in multi-centre clinical trials where study participants are randomized to different treatment groups. The system generates a unique randomization code for each participant, which is used to assign them to a treatment group.
The ARS IWRS system also allows study personnel to monitor the progress of the study, track participant enrolment and study visits, and manage the drug supply and distribution.
ARS IWRS system is designed to be user-friendly and accessible to study personnel at all levels of the study organization. The system is often integrated with other clinical trial software systems, such as electronic data capture (EDC) systems and clinical trial management systems (CTMS), to provide a comprehensive solution for managing clinical trials.
Features of ARS IWRS

Randomization
ARS IWRS system allows for the random assignment of participants to different treatment groups or study arms. This ensures that the allocation of participants is unbiased and helps to minimize any potential confounding factors.

Treatment Allocation
Based on the randomization process, the ARS IWRS system assigns participants to specific treatment groups and provides treatment codes or identifiers that are used to dispense the correct medication to the participants.

Study Compliance
ARS IWRS system ensures compliance with regulatory requirements and helps maintain the integrity of the study. It provides an audit trail of all system activities and allows for monitoring and resolving data discrepancies.

Study Monitoring
ARS IWRS enables real-time monitoring and oversight of the study. The central study management team can track participant enrolment, drug supply status, and study progress. It also allows for prompt intervention in case of any issues or deviations from the study protocol.

Blinding and Masking
ARS IWRS system can support blinding and masking procedures in a study. It ensures that study participants, investigators, and data analysts are kept unaware of the treatment assignments, minimizing bias in the study results.

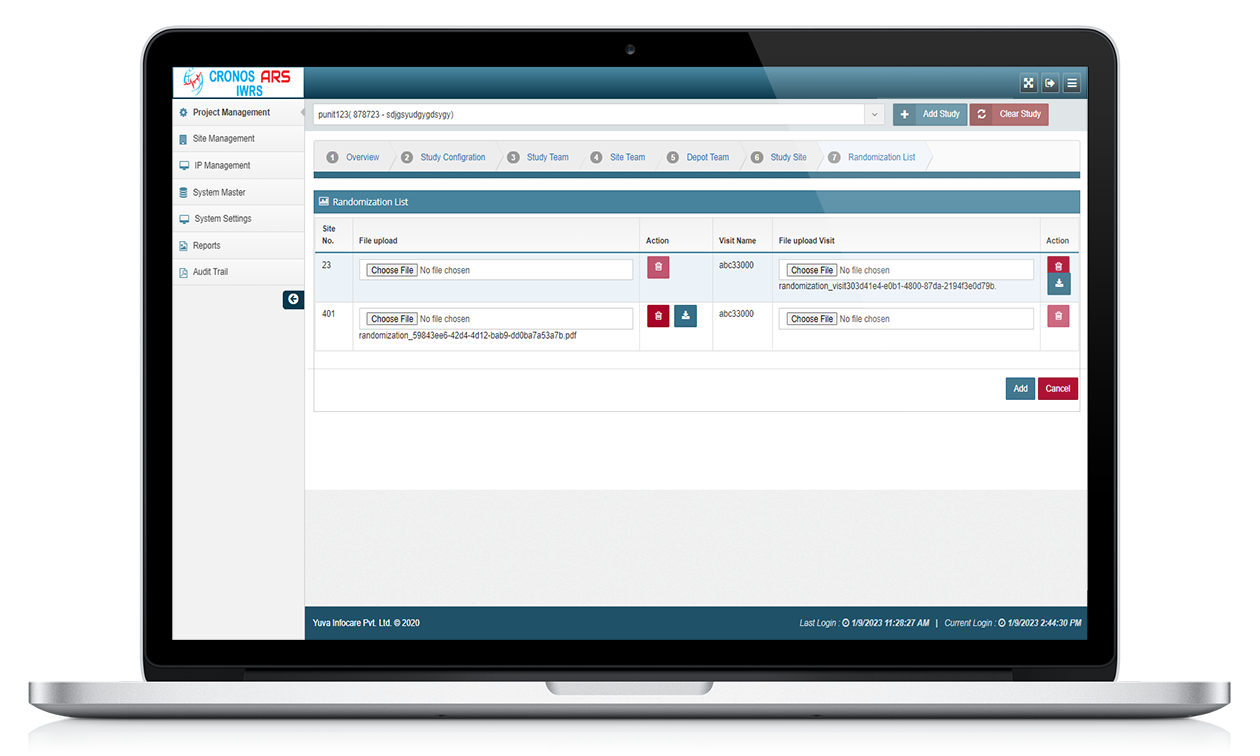
Drug Supply Management
The system helps in managing the drug supply and distribution throughout the study. It keeps track of drug inventory, shipping, and re-supply. The system can also provide drug accountability reports to ensure accurate tracking of medication usage.

Audit trails
ARS IWRS systems can track changes made to data, providing a record of who made changes and when they were made. ARS IWRS systems can provide an audit trail of all data changes, ensuring that the data is traceable and secure.
Overall, the ARS IWRS system plays a crucial role in managing the complexities of clinical trials by providing an automated and centralized platform for randomization, drug supply management, patient tracking, and study management. It enhances efficiency, accuracy, and regulatory compliance in the conduct of clinical research studies.
ARS eTMF
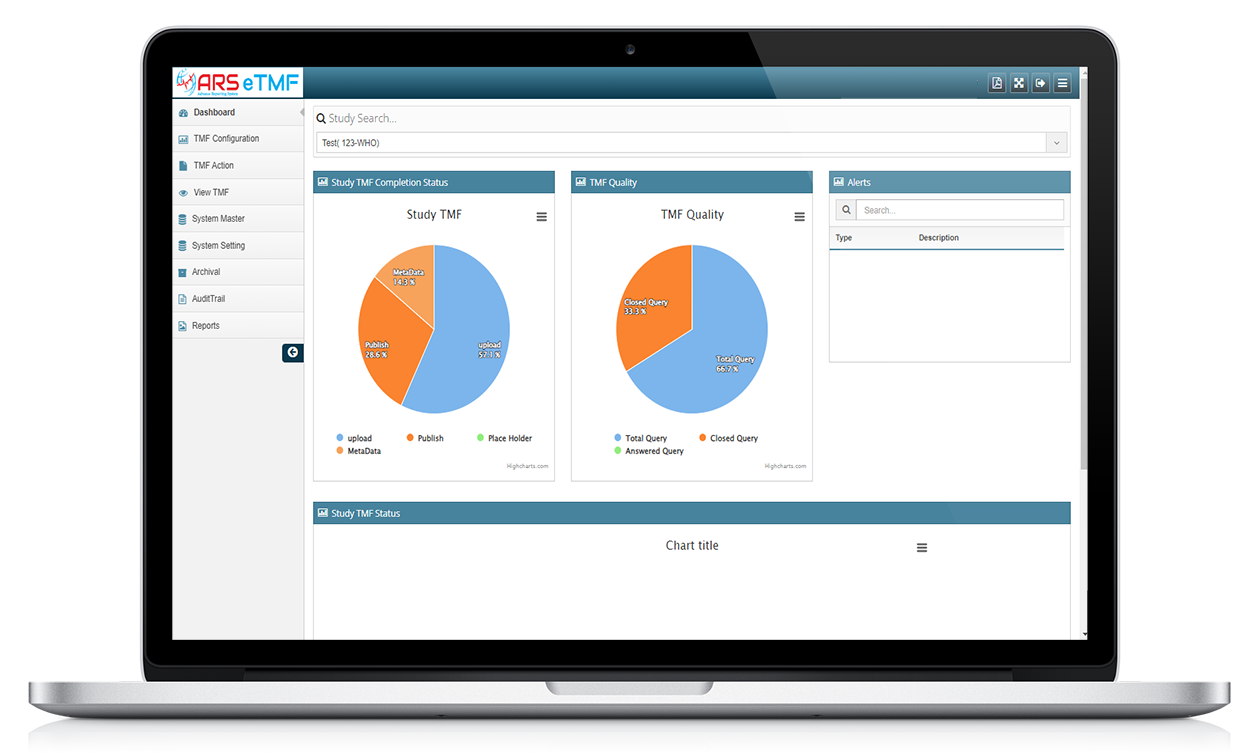
Features of ARS eTMF
Document Management
ARS eTMF system provides a centralized repository for all trial-related documents, including protocols, study reports, regulatory documents, and case report forms.

Regulatory Compliance
ARS eTMF system ensures compliance with regulatory requirements, such as the FDA's 21 CFR Part 11 and the European Medicines Agency's (EMA) Good Clinical Practice (GCP) guidelines. It facilitates the organization and assists in presentation of trial documents during regulatory inspections and audits.

Audit Trial
ARS eTMF provides a comprehensive audit trail and supports electronic signatures, enabling a paperless trial environment. Audit trails captures each and all user activities and modifications made to documents time to time.
Workflow Management
The system allows for the creation and tracking of document workflows, enabling efficient collaboration between study team members and document authors. It provides notifications and reminders for document reviews, approvals, and signatures, ensuring that documents are processed in a timely and compliant manner
ARS eTMF allows for document upload, indexing, and version control, ensuring that the most up-to-date version is available to all authorized users. Documents are organized into electronic folders or categories, making it easier to locate and retrieve specific information.

Collaboration and Remote Access
ARS eTMF systems enable efficient collaboration among study team members, sponsors, and stakeholders. Authorized users can access the system remotely, allowing for real-time document sharing, review, and collaboration regardless of geographical locations.

Security and Access Control
ARS eTMF system implements robust security measures to protect the integrity and confidentiality of trial documents. It allows for user authentication, role-based access control, and encryption of sensitive data.
ARS eTMF system provides secure access to trial documents and controls user permissions based on predefined roles and responsibilities. It ensures that documents are accessed only by authorized personnel, and tracks all user activities to maintain an audit trail.
By adopting Yuva’s ARS eTMF solution, organizations involved in clinical trials can streamline document management processes, enhance collaboration, improve document quality and completeness, and ensure regulatory compliance. It facilitates efficient access to trial documentation, ultimately supporting the successful execution of clinical research studies.
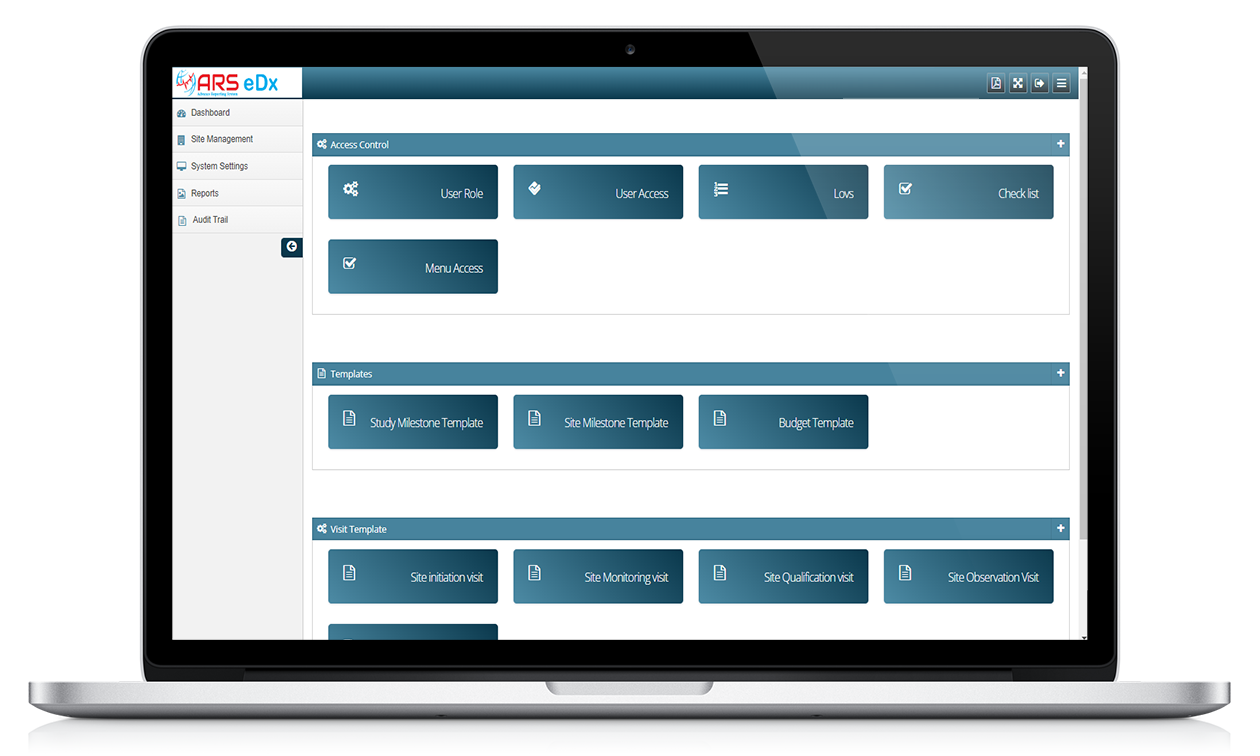
ARS eDX
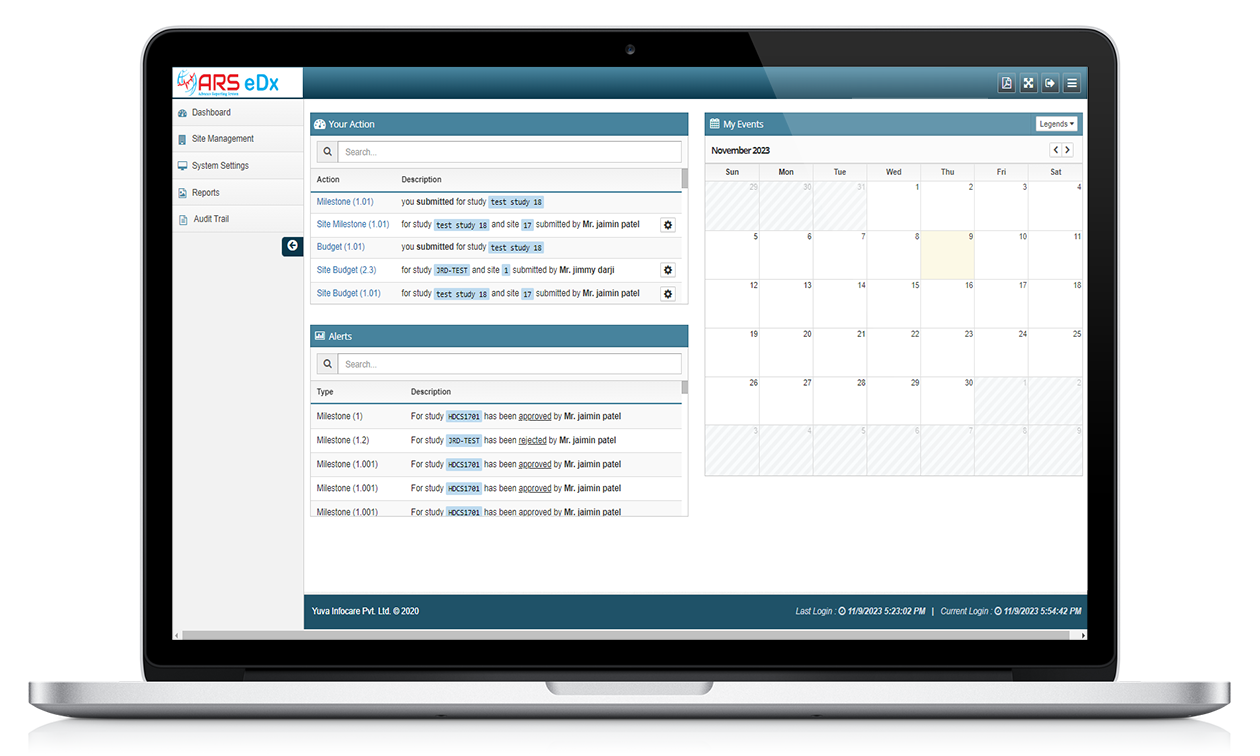
Features of ARS eDX
Remote Monitoring
With ARS eDX, monitors or data managers access electronic data and documents remotely, typically through a secure web-based system, to review and verify source data collected at the investigative sites.
Risk-Based Monitoring
ARS eDX is often employed as part of risk-based monitoring strategies in clinical trials. Risk assessment is conducted to identify critical data and high-risk areas, allowing monitors to focus their efforts on those specific data points during remote reviews.
Data Review and Query Management
The ARS eDX system provides tools for data review and query management. Monitors or data managers can review electronic source data, compare it with the entered data, and raise queries or clarifications to investigative sites electronically. The system tracks and manages the query resolution process, ensuring timely responses and documentation.

Collaboration and Communication
The system facilitates communication and collaboration between monitors, data managers, and investigative sites. It enables secure messaging, document sharing, and real-time notifications to ensure effective and efficient communication during the remote data verification process.
Data Validation
During remote source data verification and exchange, monitors or data managers compare the source data against the data entered into the EDC system. They review the data for accuracy, completeness, and compliance with study protocols, ensuring the integrity of the clinical trial data.
Data Access and Integration
ARS eDX system integrates with electronic data capture (EDC) systems or other data management platforms to access and retrieve study data remotely. It enables real-time or near real-time access to electronic case report forms (eCRFs), patient data, and relevant study documentation.
Reporting and Analytics
ARS eDX system generates reports and provides analytics on the progress and outcomes of the remote data verification activities. It provides insights into the quality of data, query resolution rates, and compliance metrics, supporting data-driven decision-making and monitoring of study progress.

Regulatory Compliance
ARS eDX system adheres to regulatory requirements and industry guidelines, such as Good Clinical Practice (GCP) and data integrity standards. It ensures that the remote data verification process follows established protocols, maintains data quality, and supports compliance with regulatory obligations.
ARS eDX offers several benefits, including reduced travel costs and time associated with on-site monitoring visits, improved efficiency, and the ability to monitor larger and geographically dispersed clinical trials.
However, it is important to consider the nature of the trial, the complexity of the data, and regulatory requirements when determining the appropriateness of eDX for a particular study which will be assisted by Yuva team members.
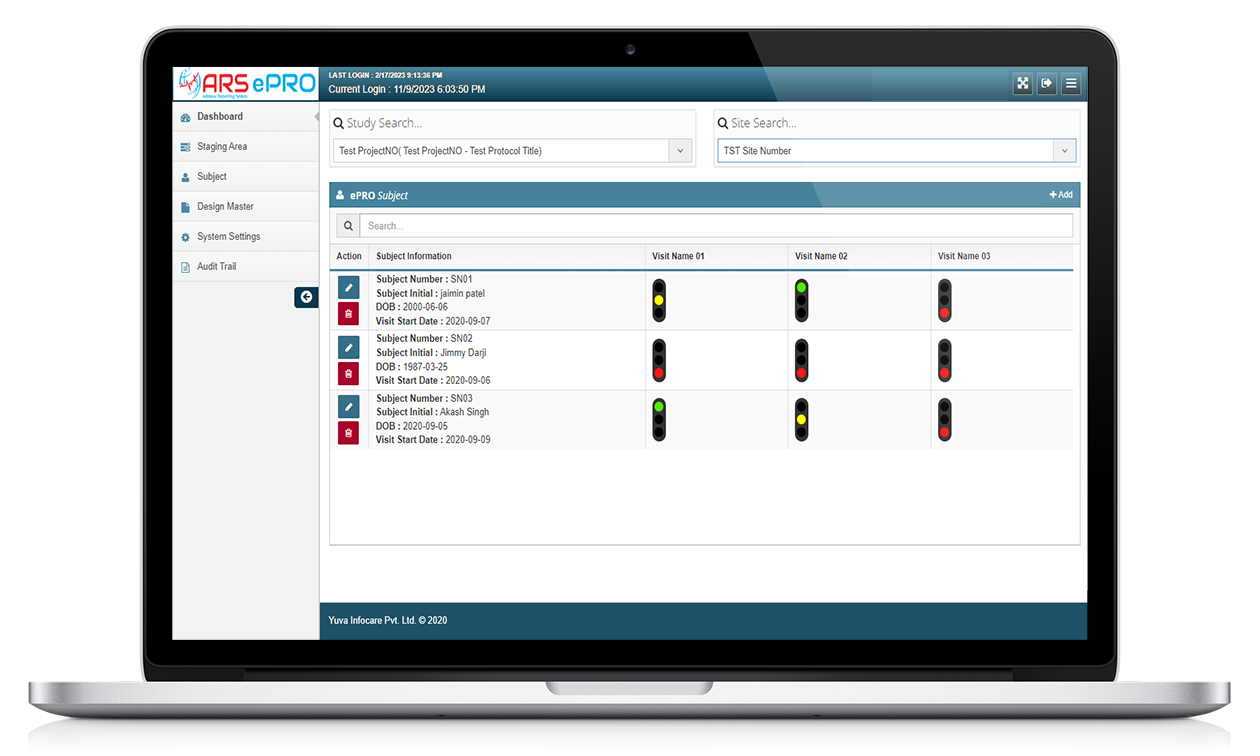
ARS ePRO
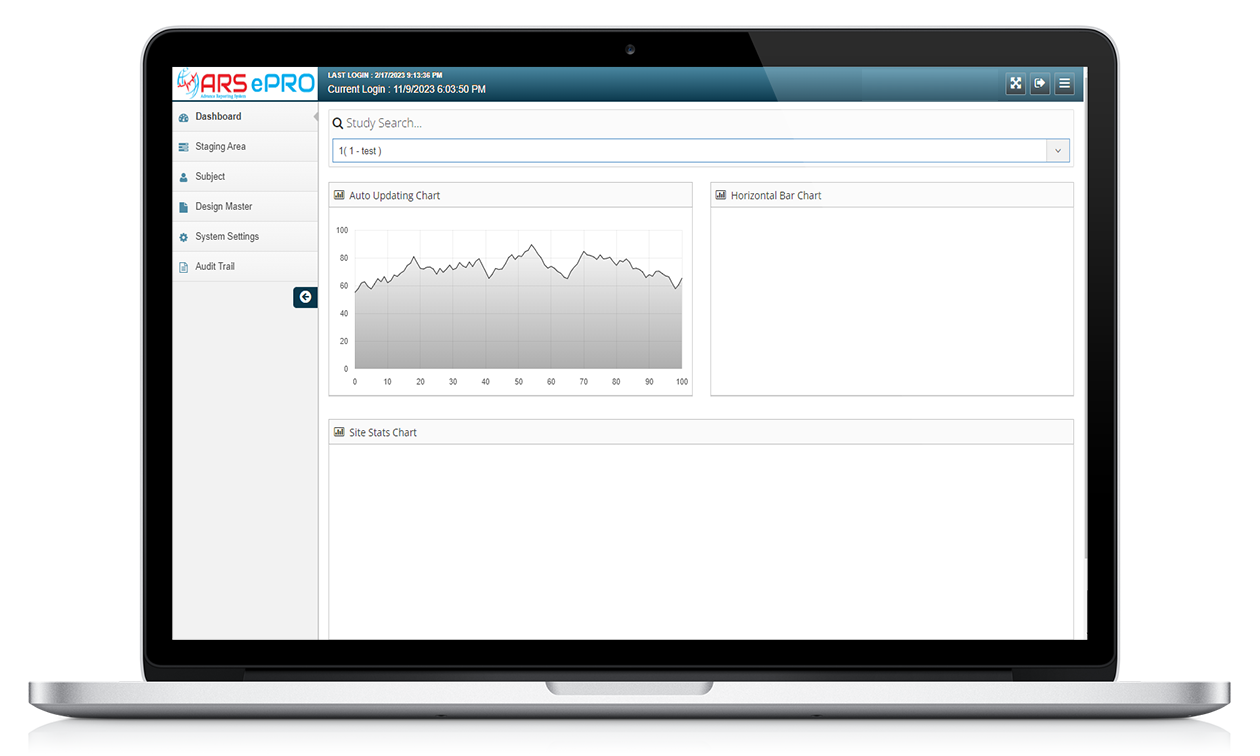
ARS ePRO (electronic Patient-Reported Outcome) system by Yuva Infocare Pvt Ltd is a digital platform and innovative tool used in clinical trials and healthcare settings to collect patient-reported data electronically. It enables patients to report their symptoms, experiences, and other relevant information directly into a digital interface in intuitive manner using ARS ePRO solution.
It is an innovative approach to clinical data collection that improves the accuracy and completeness of patient-reported outcomes, which can be critical in clinical trials, observational studies, and other healthcare research.
Features of ARS ePRO
Electronic Data Capture
Customizable Assessments


Real-time Data Capture
ARS ePRO system captures patient-reported data in real-time, eliminating the need for manual data entry and reducing the risk of errors or missing data. This feature ensures that the data is collected consistently and accurately throughout the study.
User-friendly Interface
ARS ePRO system offers a user-friendly interface for patients to complete assessments easily and efficiently. The interface is designed to be intuitive and user-friendly, providing clear instructions and feedback to patients to ensure accurate data collection.
Patient Engagement and Compliance


Compliance and Regulatory Considerations
ARS ePRO system is designed to comply with regulatory requirements and industry standards, such as Good Clinical Practice (GCP). They may incorporate electronic signatures, audit trails, and data encryption to ensure data integrity and security.
Data Integration and Analysis
ARS ePRO system can integrate with other clinical trial or healthcare information systems, such as electronic health records (EHR) or clinical data management system (EDC). This enables seamless data transfer, integration, and analysis for a comprehensive view of patient data.
Healthcare Regulatory Compliance Solutions: -
Healthcare is one of the fastest developing domains with numerous breakthrough discoveries. Healthcare is a sector that provides goods and services to treat patients with curative, preventive or rehabilitative care.
Yuva Infocare as a Life Science Technology company, focused on providing Pharmaceutical, Multivigilance, Med Device Vigilance, Cosmetovigilance, Nutrivigilance Technology products and solutions to the Pharmaceutical Industry. We have been working with Pharma companies for over years, developing custom solutions, websites and providing product development services.

Yuva Infocare has a team of domain experts who work alongside technology specialists. Client requirements are clearly understood and translated into software. Our domain knowledge stands out in good stead, when providing précised, customized and with scope of development solutions like Pharmacovigilance software, Clinical Data Management, Post Marketing Clinical Trail, Expense Tracking Systems, Closed Loop Marketing Systems, Mobile applications, Sales Reporting and CRM solutions.
With in depth knowledge of ISO, GAMP5, US FDA 21, EU Annex 11, GxP, 21 CFR part 11 standards, Risk based Software Validations, Yuva Infocare Pvt Ltd offers its services in development of regulatory compliant solutions.
Yuva Infocare Pvt Ltd has expertise to develop regulatory compliance solutions with an integrated approach to meet global regulatory standards.
Our solutions support regulatory compliance in software development through strict SDLC, Change Request, Document control, Training, Audits, RCA, CAPA…
Practices at Yuva Infocare Pvt Ltd have made software development a task which follows compliance through computer systems validation (CSV) for software development and implementation.

