* In today’s fast-paced world of clinical research, managing trials efficiently isn’t just important, it’s essential.
* With growing regulatory demands and the complexities of multiple sites, research teams need smarter, faster, and more reliable ways to keep up.
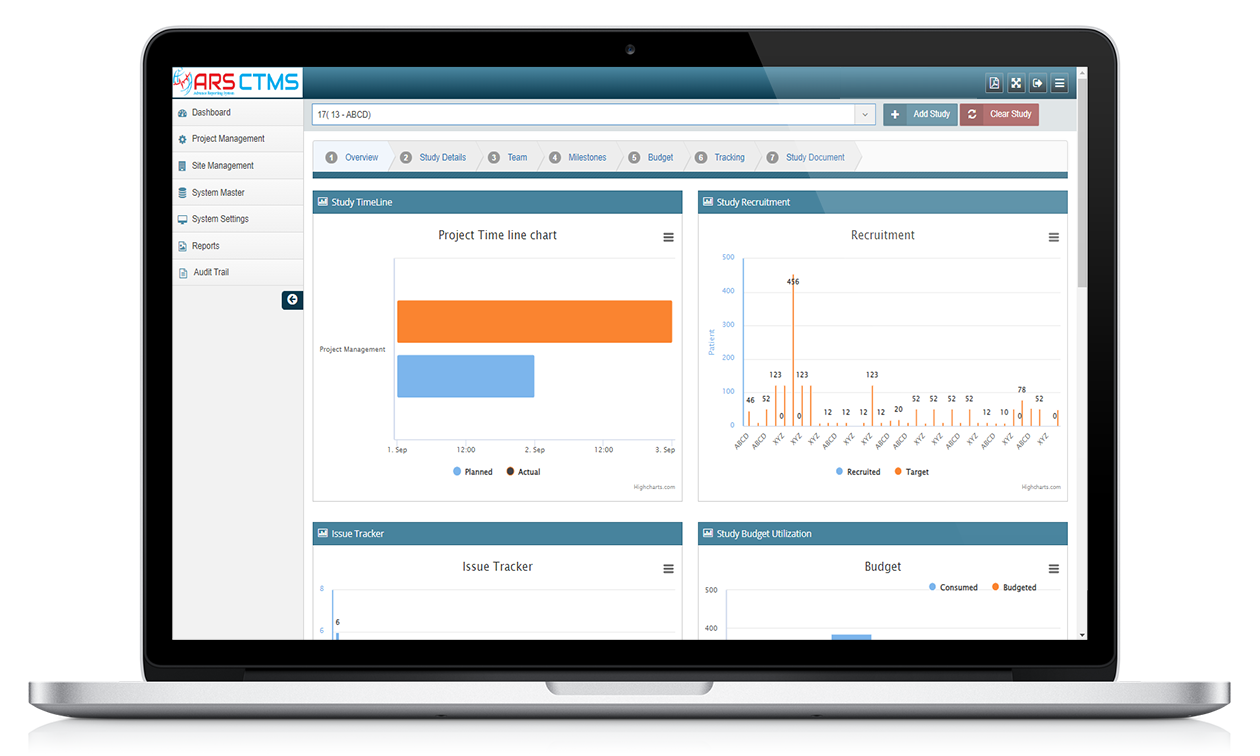
* That’s where ARS CTMS comes in. This powerful, cloud-based clinical trial management system is designed to simplify processes and increase success
What is ARS CTMS?
* More than just software, ARS CTMS is a clinical operations solution created by Yuva Infocare. It draws on the hands-on insights of professionals with real-world experience.
* Unlike traditional systems that often don’t meet needs, ARS CTMS is built to address the challenges faced by sponsors, CROs, and research sites.
* Whether you’re conducting a small investigator-led study or managing a large, multi-center trial, ARS CTMS offers an intuitive platform to manage every step, from protocol planning to final reporting.