ARS CTMS
At Yuva Infocare, our Clinical Operations domain experts collaborated to identify the core challenges and requirements in clinical trial management systems. Through extensive industry insights and hands-on experience, our team analyzed the limitations of existing CTMS platforms and designed a solution that addresses these gaps effectively.
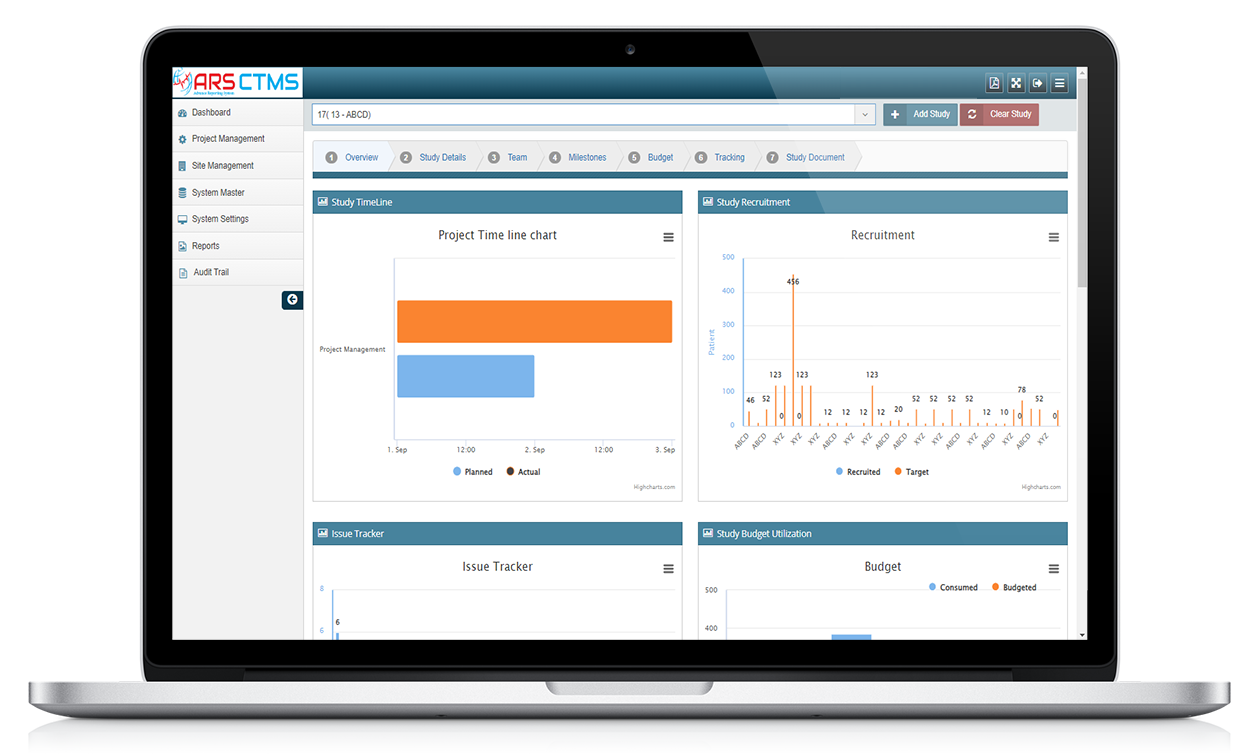
The result is ARS CTMS – a robust, cloud-based Clinical Trial Management System that supports end-to-end clinical trial activities with automation, integration, and real-time access.
ARS CTMS is engineered to streamline and centralize all critical trial operations, ensuring enhanced collaboration, transparency, and compliance. From protocol development to site management, and from subject recruitment to financial tracking, the platform offers an intuitive interface and powerful features to manage every aspect of clinical trials efficiently.
...
Key functionalities include:
Protocol and Study Design Management: Simplify the setup and configuration of study protocols and timelines.
Subject Recruitment and Enrollment: Accelerate participant onboarding through intelligent tracking and engagement tools.
Site and Investigator Management: Monitor site performance, staff assignments, and compliance across multiple locations.
Document and Data Management: Securely store, share, and access essential trial documents and datasets.
Visit Scheduling and Workflow Automation: Ensure seamless coordination of patient visits and trial milestones.
Real-time Reporting and Analytics: Gain actionable insights through customizable dashboards and reports.
Financial and Budget Tracking: Track trial budgets, site payments, and financial metrics accurately.
By providing a centralized digital platform, ARS CTMS helps research organizations, CROs, and sponsors maintain operational efficiency, regulatory compliance, and data integrity throughout the lifecycle of a clinical trial.
Whether you’re managing a single-site study or a complex multi-center trial, ARS CTMS offers the flexibility and scalability needed to support your clinical research goals.
Read more