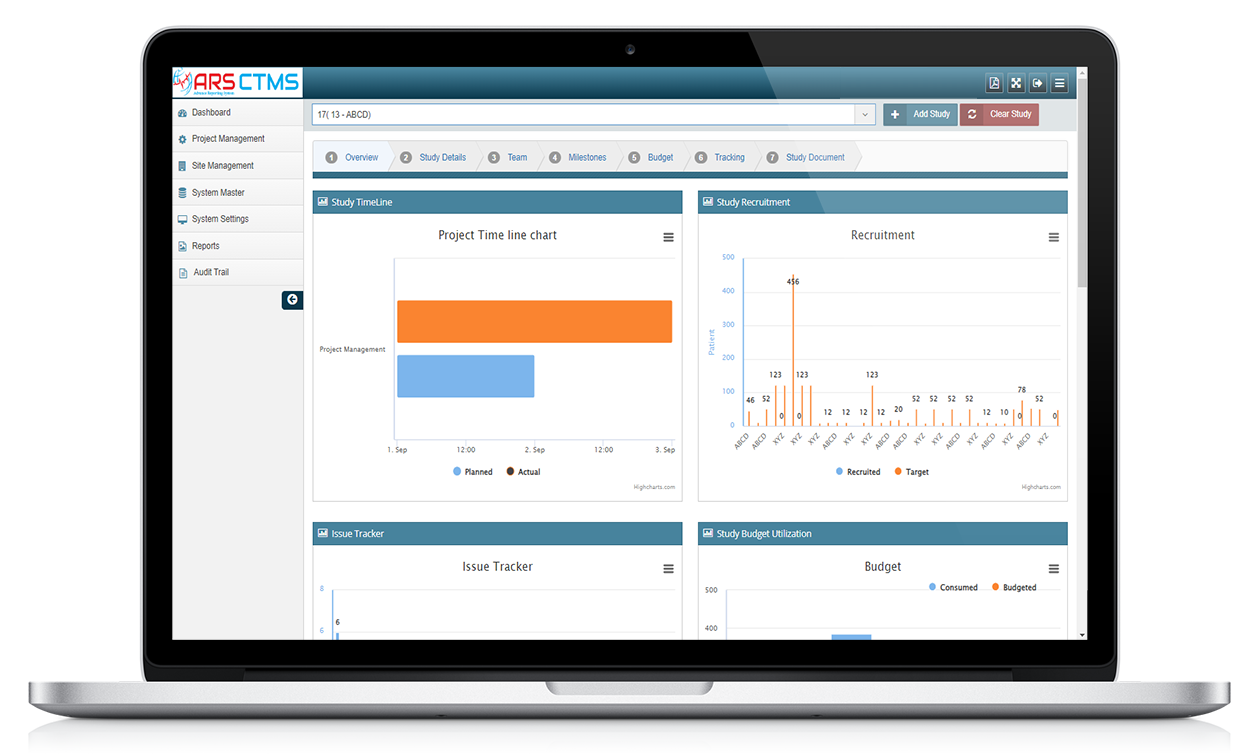
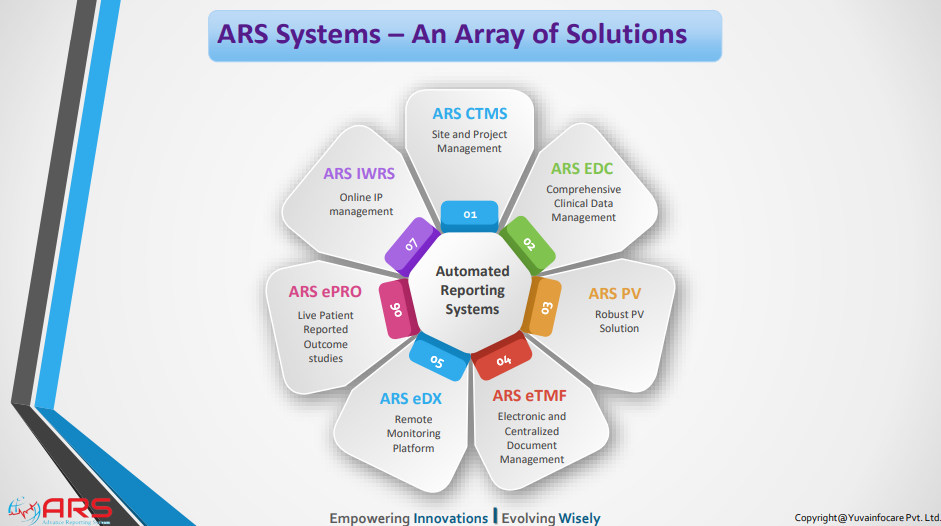
ARS CTMS
Protocol management

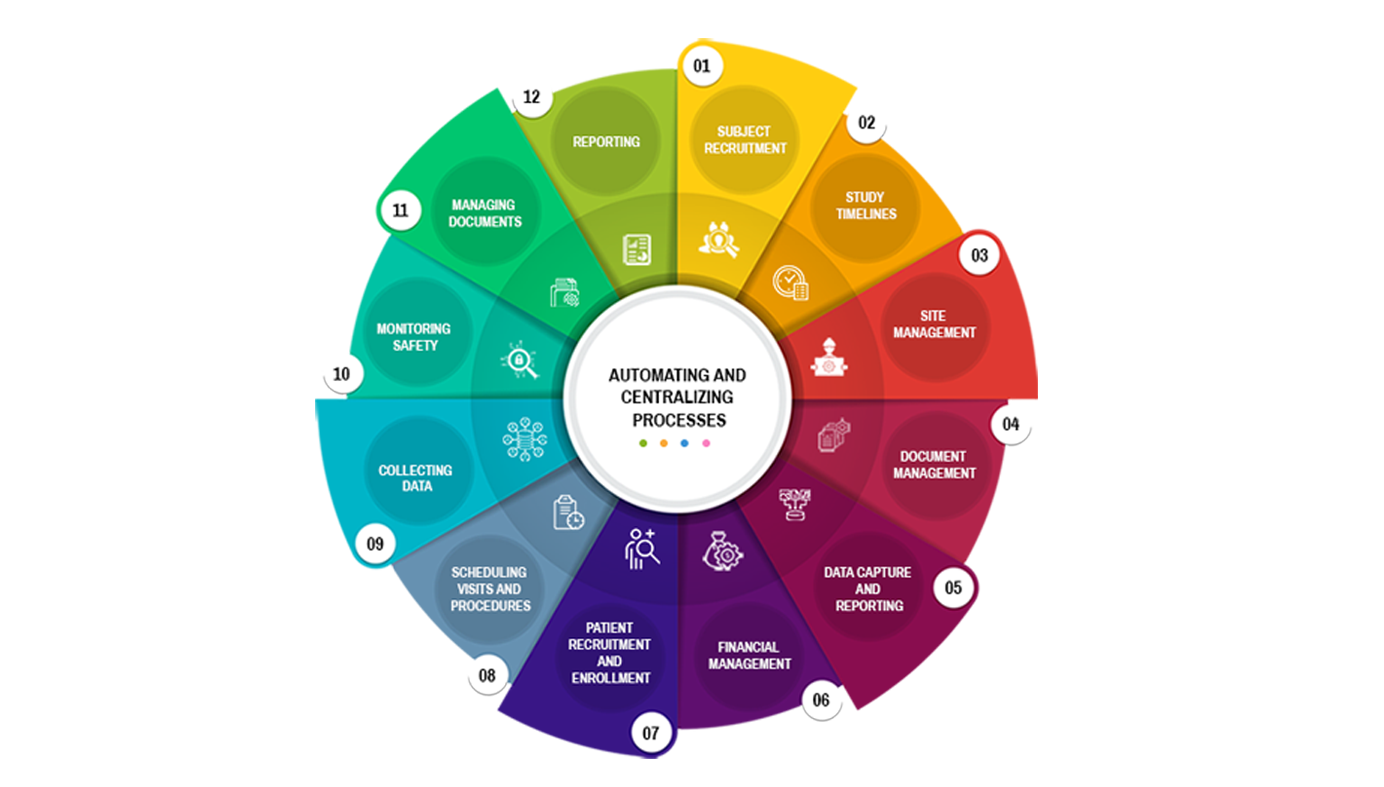
Subject recruitment and management
ARS CTMS can help manage the different study sites by providing tools to manage site initiation, site monitoring, and site closeout activities.
Site management
Document management
ARS CTMS can help manage the different documents associated with a clinical trial by providing tools to store, track, and share documents securely.
Data capture and reporting
Financial management
ARS CTMS can help manage the financial aspects of a clinical trial by providing tools to track expenses, payments, and budgets.
Compliance and regulatory management

Subject recruitment and management
ARS CTMS can help manage the recruitment of subjects by providing tools to screen, enrolment, and track the progress of study participants.

