ARS EDC/ ARS eCRF
ARS EDC stands for Electronic Data Capture by Advance Reporting System powered by Yuva Infocare Pvt Ltd, which is a computerized system used in clinical trials to capture, manage, and report clinical trial data. ARS EDC replaces traditional paper-based data collection methods, making the process more efficient and accurate.
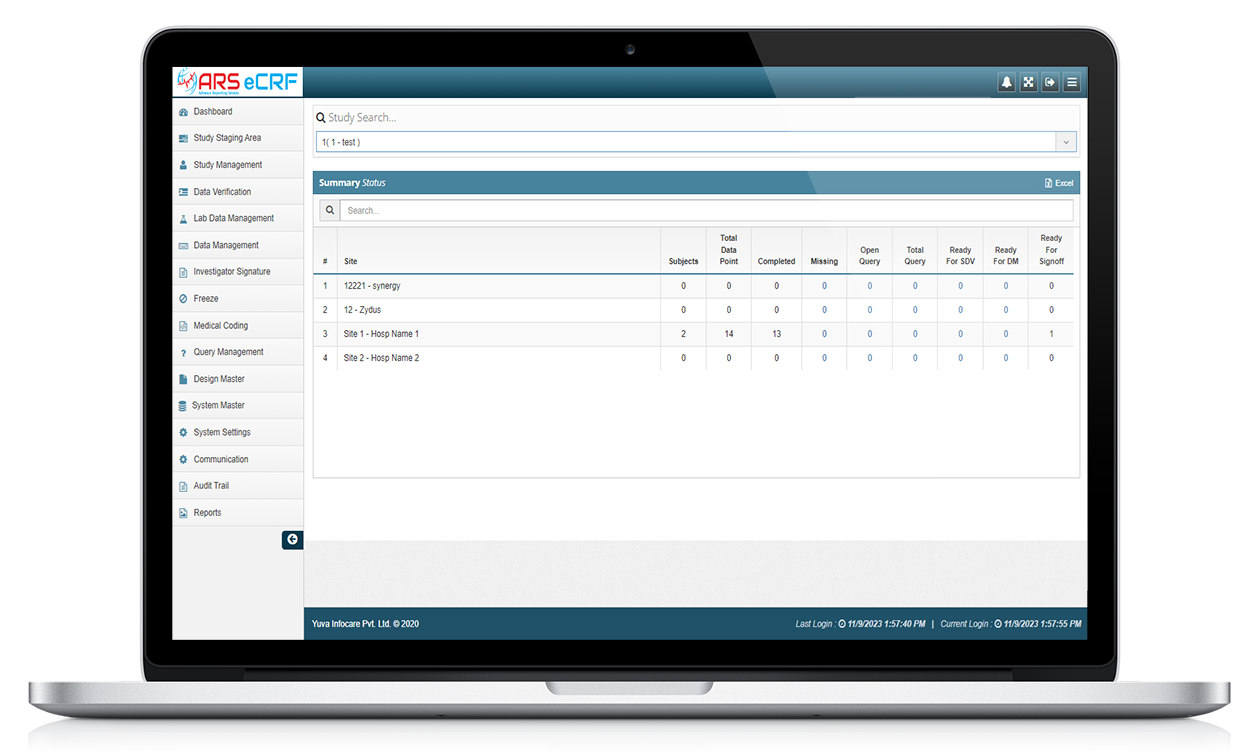
ARS EDC system allow for the electronic collection of clinical trial data, which can be entered directly into the system by the investigator or study coordinator, or through direct data capture from medical devices. ATS EDC system typically have features such as data validation and real-time data monitoring to ensure the accuracy and completeness of the data collected.
Some features of ARS EDC systems include
Data entry
ARS EDC systems provide electronic forms for entering data, replacing traditional paper-based forms.
Data monitoring
ARS EDC systems can monitor data entered into the system, alerting users to any data discrepancies or missing data. ARS EDC systems can provide real-time monitoring of study data, allowing for early identification of data anomalies or protocol violations. This can help reduce the risk of errors and ensure compliance with study protocols.
Remote monitoring
ARS EDC systems can enable remote monitoring of study data, allowing sponsors and monitors to access study data securely from anywhere in the world. This can help reduce the time and cost associated with on-site monitoring visits.
Data export
ARS EDC systems can export data in various formats, making it easy to share data with other researchers or regulatory agencies. This can facilitate the analysis and interpretation of study data.
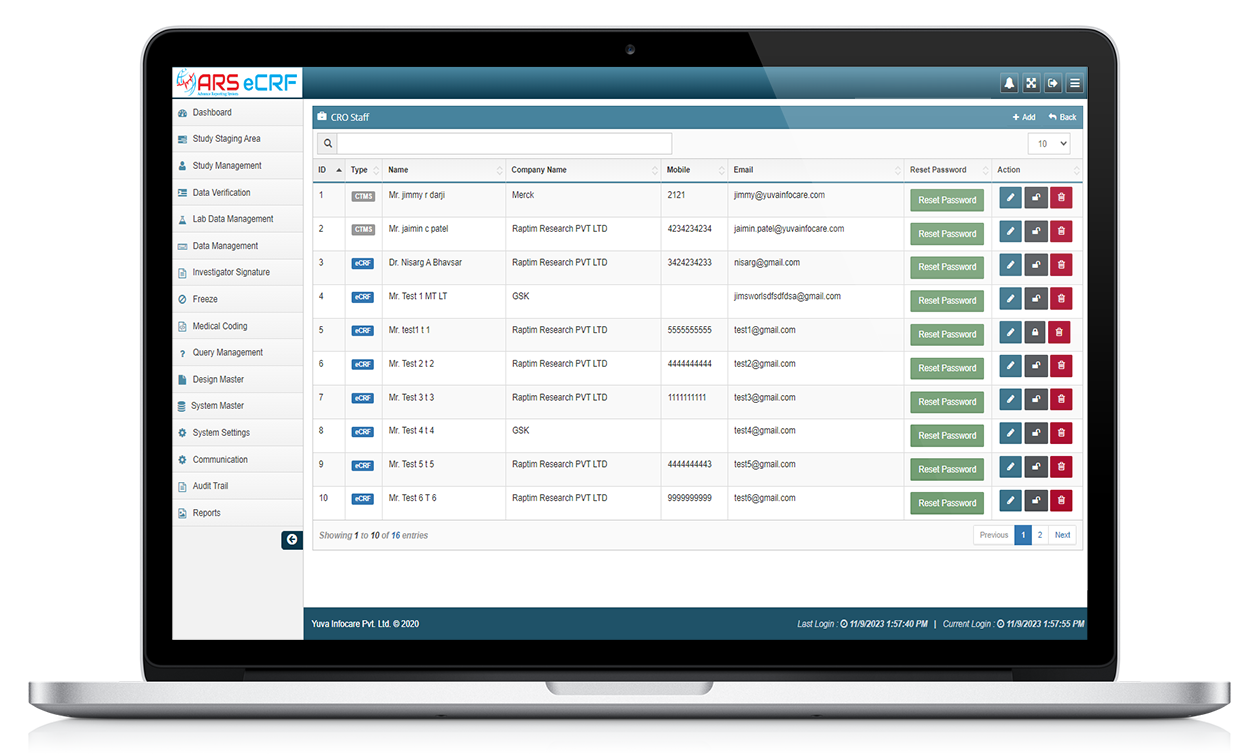
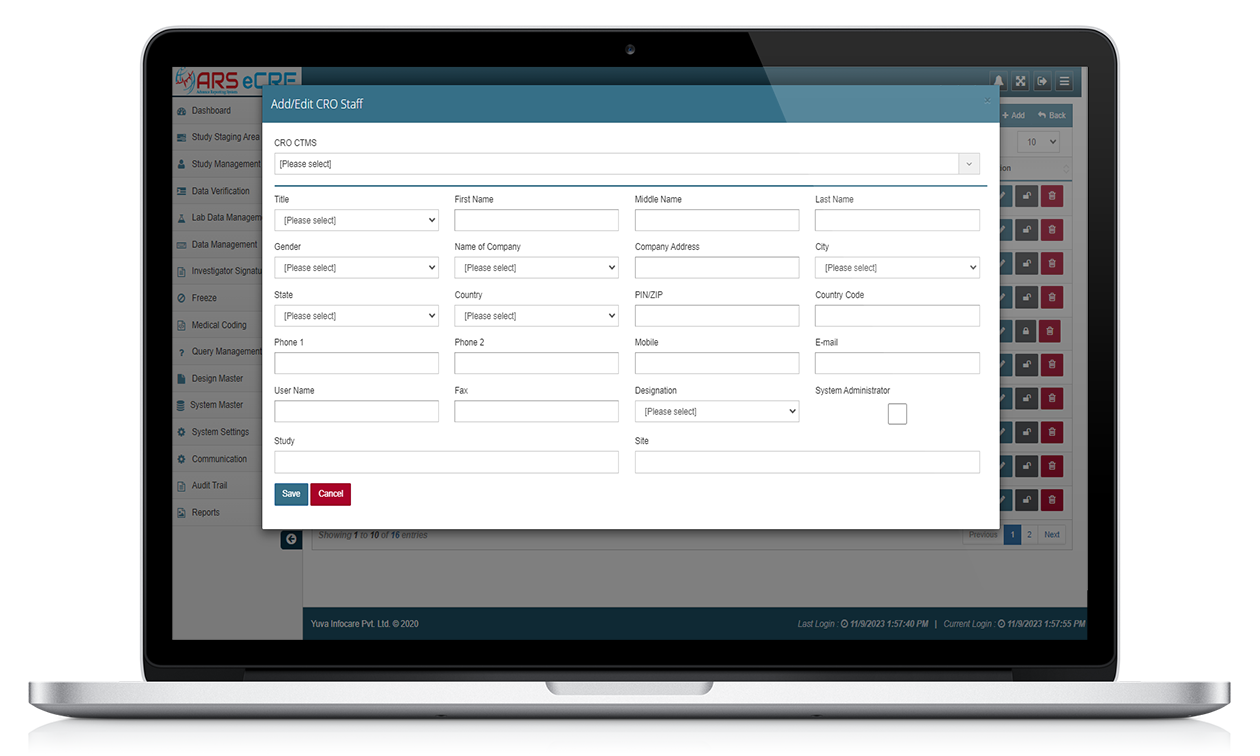
Overall, ARS EDC systems provide a more efficient and accurate way to collect and manage clinical trial data. They can improve data quality, reduce data entry errors, and provide real-time data monitoring, making them an essential tool for clinical trial management.
ARS EDC systems provide a powerful tool for the collection, management, and analysis of clinical trial data, improving efficiency, accuracy, and compliance in clinical research.

