ARS eDX
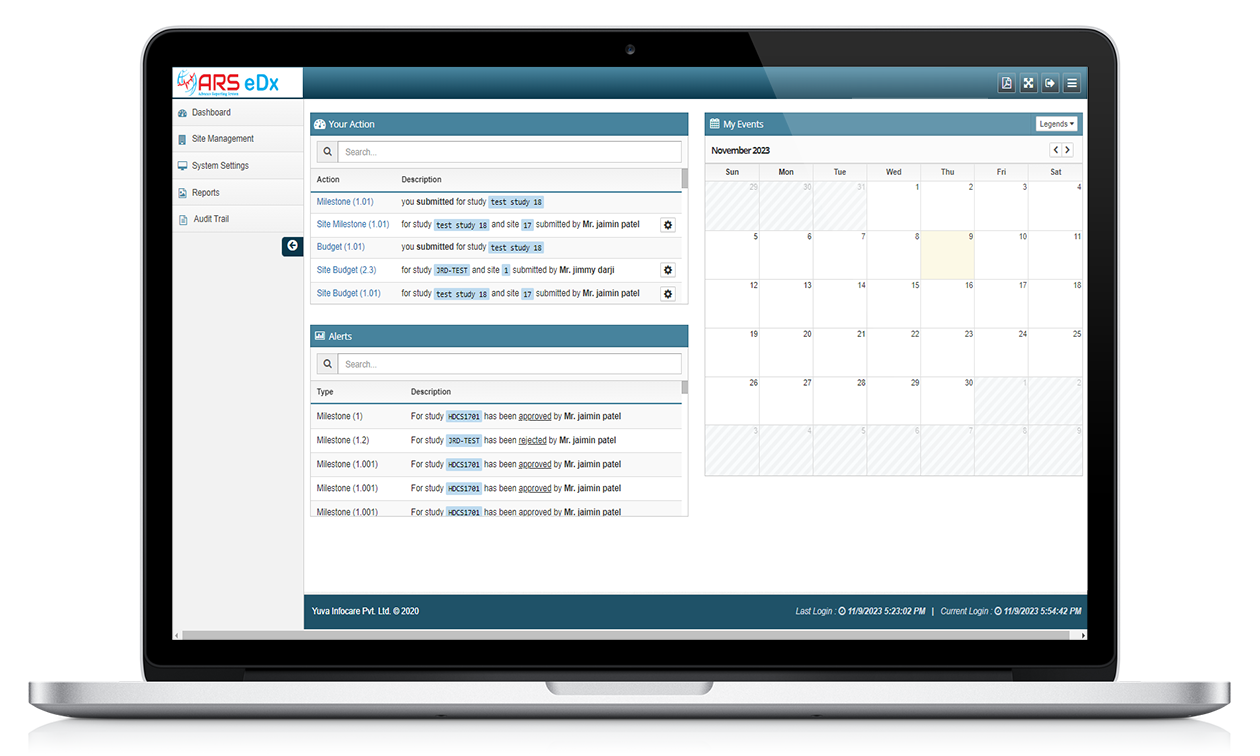
Features of ARS eDX
Remote Monitoring
With ARS eDX, monitors or data managers access electronic data and documents remotely, typically through a secure web-based system, to review and verify source data collected at the investigative sites.
Risk-Based Monitoring
ARS eDX is often employed as part of risk-based monitoring strategies in clinical trials. Risk assessment is conducted to identify critical data and high-risk areas, allowing monitors to focus their efforts on those specific data points during remote reviews.
Data Review and Query Management
The ARS eDX system provides tools for data review and query management. Monitors or data managers can review electronic source data, compare it with the entered data, and raise queries or clarifications to investigative sites electronically. The system tracks and manages the query resolution process, ensuring timely responses and documentation.

Collaboration and Communication
The system facilitates communication and collaboration between monitors, data managers, and investigative sites. It enables secure messaging, document sharing, and real-time notifications to ensure effective and efficient communication during the remote data verification process.
Data Validation
During remote source data verification and exchange, monitors or data managers compare the source data against the data entered into the EDC system. They review the data for accuracy, completeness, and compliance with study protocols, ensuring the integrity of the clinical trial data.
Data Access and Integration
ARS eDX system integrates with electronic data capture (EDC) systems or other data management platforms to access and retrieve study data remotely. It enables real-time or near real-time access to electronic case report forms (eCRFs), patient data, and relevant study documentation.
Reporting and Analytics
ARS eDX system generates reports and provides analytics on the progress and outcomes of the remote data verification activities. It provides insights into the quality of data, query resolution rates, and compliance metrics, supporting data-driven decision-making and monitoring of study progress.

Regulatory Compliance
ARS eDX system adheres to regulatory requirements and industry guidelines, such as Good Clinical Practice (GCP) and data integrity standards. It ensures that the remote data verification process follows established protocols, maintains data quality, and supports compliance with regulatory obligations.
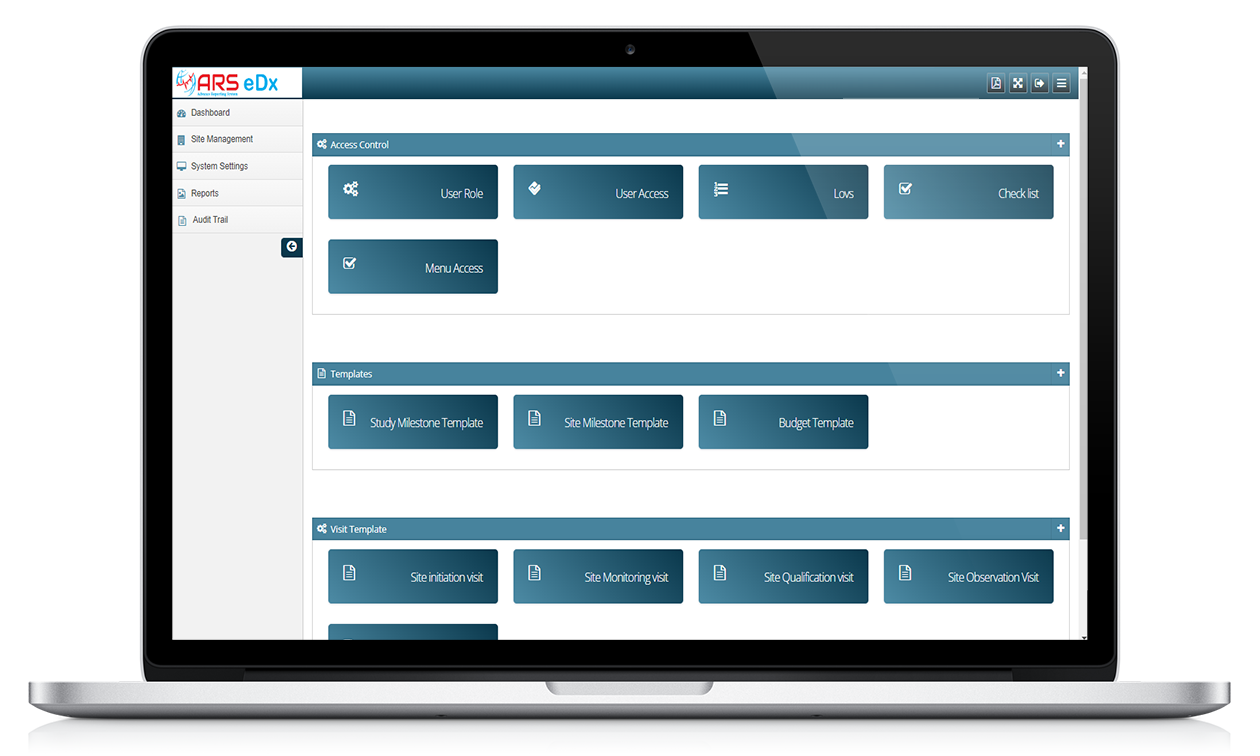
ARS eDX offers several benefits, including reduced travel costs and time associated with on-site monitoring visits, improved efficiency, and the ability to monitor larger and geographically dispersed clinical trials.
However, it is important to consider the nature of the trial, the complexity of the data, and regulatory requirements when determining the appropriateness of eDX for a particular study which will be assisted by Yuva team members.

