ARS IWRS
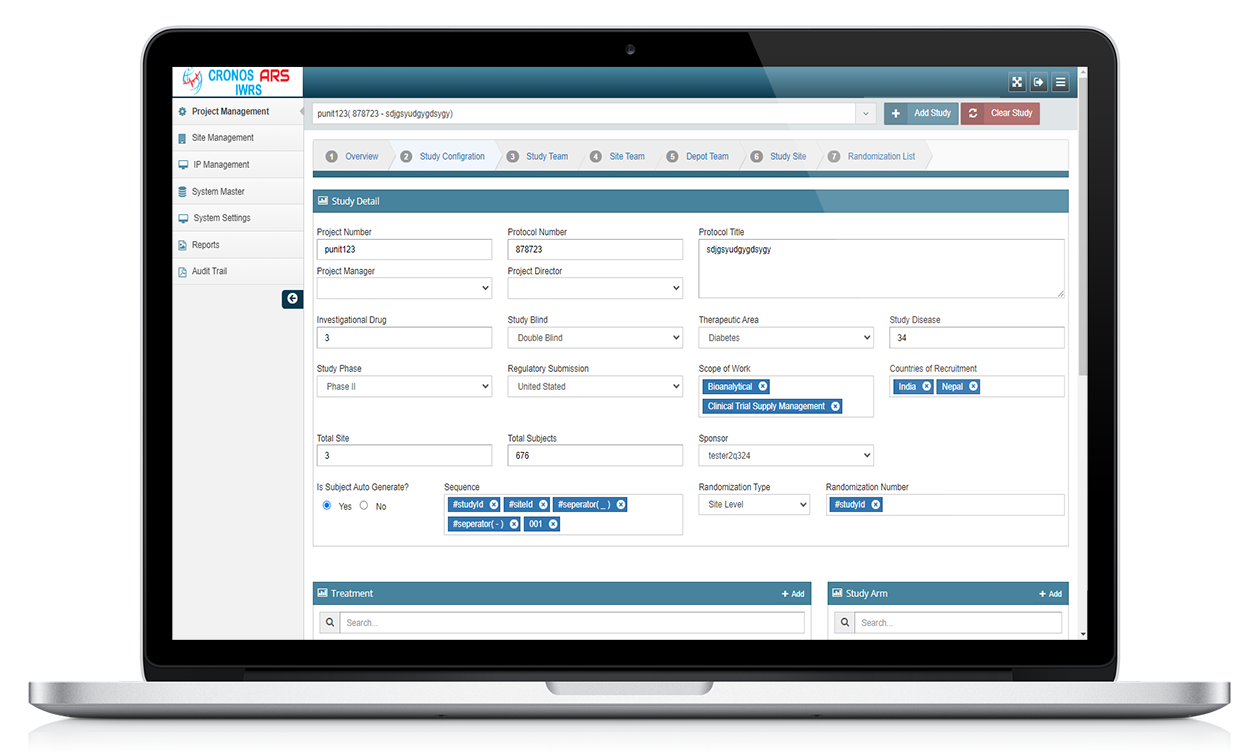
ARS IWRS stands for Interactive Web Response System powered by Yuva Infocare Pvt Ltd. It is a computerized system used in clinical trials and research studies to manage and control the randomization and drug supply processes.
The ARS IWRS system is typically accessed through a secure web portal and provides real-time interactions between the study site and the central study management team.
ARS IWRS system is typically used in multi-centre clinical trials where study participants are randomized to different treatment groups. The system generates a unique randomization code for each participant, which is used to assign them to a treatment group.
The ARS IWRS system also allows study personnel to monitor the progress of the study, track participant enrolment and study visits, and manage the drug supply and distribution.
ARS IWRS system is designed to be user-friendly and accessible to study personnel at all levels of the study organization. The system is often integrated with other clinical trial software systems, such as electronic data capture (EDC) systems and clinical trial management systems (CTMS), to provide a comprehensive solution for managing clinical trials.
Features of ARS IWRS

Randomization
ARS IWRS system allows for the random assignment of participants to different treatment groups or study arms. This ensures that the allocation of participants is unbiased and helps to minimize any potential confounding factors.

Treatment Allocation
Based on the randomization process, the ARS IWRS system assigns participants to specific treatment groups and provides treatment codes or identifiers that are used to dispense the correct medication to the participants.

Study Compliance
ARS IWRS system ensures compliance with regulatory requirements and helps maintain the integrity of the study. It provides an audit trail of all system activities and allows for monitoring and resolving data discrepancies.

Study Monitoring
ARS IWRS enables real-time monitoring and oversight of the study. The central study management team can track participant enrolment, drug supply status, and study progress. It also allows for prompt intervention in case of any issues or deviations from the study protocol.

Blinding and Masking
ARS IWRS system can support blinding and masking procedures in a study. It ensures that study participants, investigators, and data analysts are kept unaware of the treatment assignments, minimizing bias in the study results.

Drug Supply Management
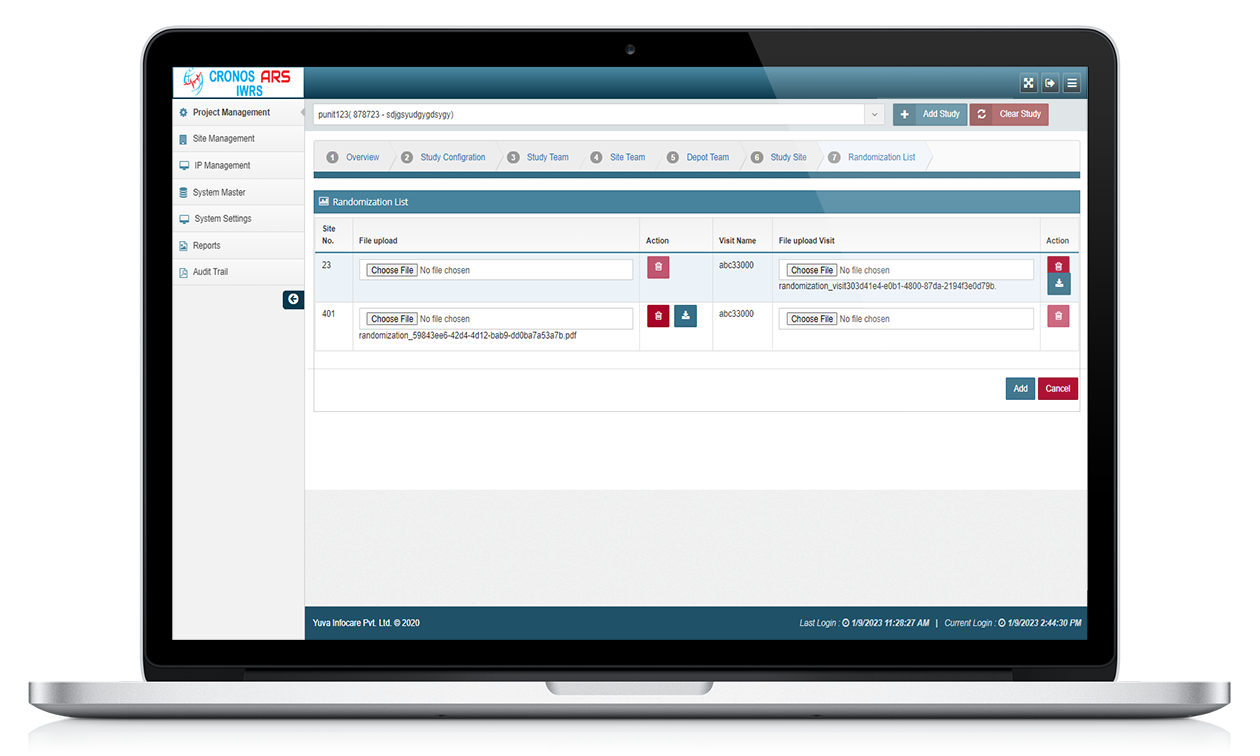
The system helps in managing the drug supply and distribution throughout the study. It keeps track of drug inventory, shipping, and re-supply. The system can also provide drug accountability reports to ensure accurate tracking of medication usage.

Audit trails
ARS IWRS systems can track changes made to data, providing a record of who made changes and when they were made. ARS IWRS systems can provide an audit trail of all data changes, ensuring that the data is traceable and secure.
Overall, the ARS IWRS system plays a crucial role in managing the complexities of clinical trials by providing an automated and centralized platform for randomization, drug supply management, patient tracking, and study management. It enhances efficiency, accuracy, and regulatory compliance in the conduct of clinical research studies.

