ARS eTMF
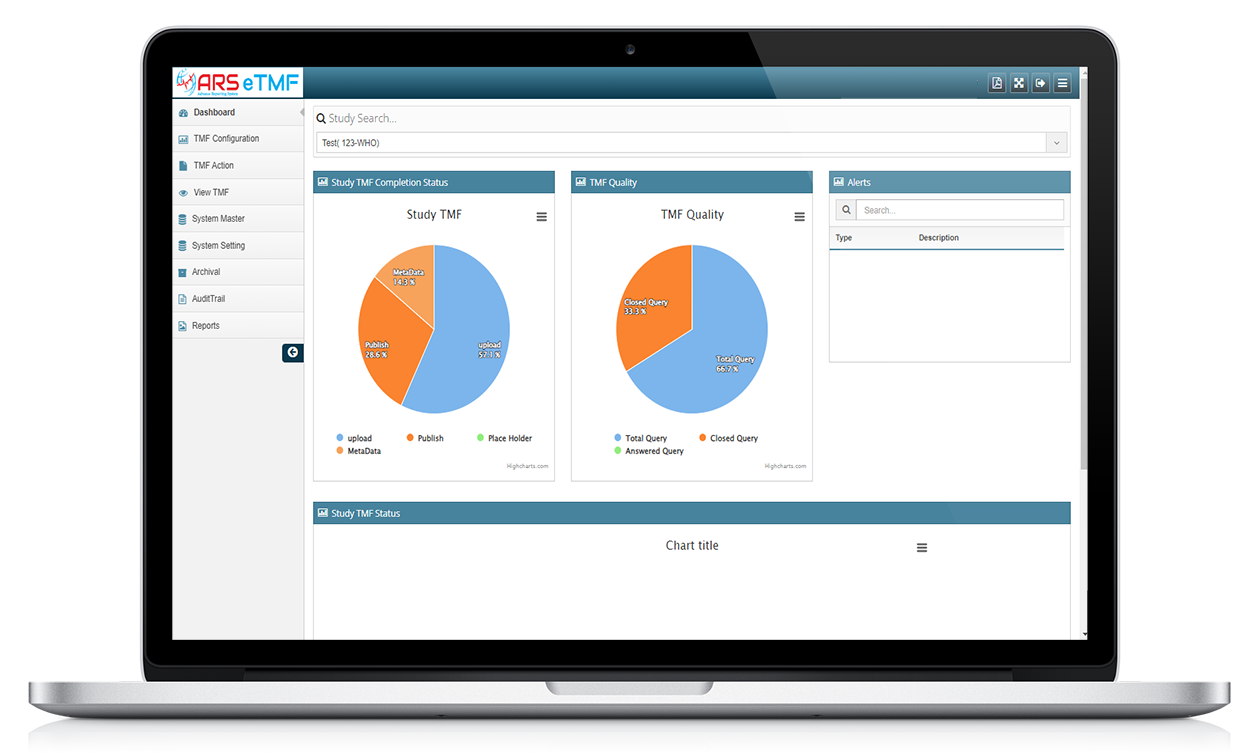
Features of ARS eTMF
Document Management
ARS eTMF system provides a centralized repository for all trial-related documents, including protocols, study reports, regulatory documents, and case report forms.

Regulatory Compliance
ARS eTMF system ensures compliance with regulatory requirements, such as the FDA's 21 CFR Part 11 and the European Medicines Agency's (EMA) Good Clinical Practice (GCP) guidelines. It facilitates the organization and assists in presentation of trial documents during regulatory inspections and audits.

Audit Trial
ARS eTMF provides a comprehensive audit trail and supports electronic signatures, enabling a paperless trial environment. Audit trails captures each and all user activities and modifications made to documents time to time.
Workflow Management
The system allows for the creation and tracking of document workflows, enabling efficient collaboration between study team members and document authors. It provides notifications and reminders for document reviews, approvals, and signatures, ensuring that documents are processed in a timely and compliant manner
ARS eTMF allows for document upload, indexing, and version control, ensuring that the most up-to-date version is available to all authorized users. Documents are organized into electronic folders or categories, making it easier to locate and retrieve specific information.

Collaboration and Remote Access
ARS eTMF systems enable efficient collaboration among study team members, sponsors, and stakeholders. Authorized users can access the system remotely, allowing for real-time document sharing, review, and collaboration regardless of geographical locations.

Security and Access Control
ARS eTMF system implements robust security measures to protect the integrity and confidentiality of trial documents. It allows for user authentication, role-based access control, and encryption of sensitive data.
ARS eTMF system provides secure access to trial documents and controls user permissions based on predefined roles and responsibilities. It ensures that documents are accessed only by authorized personnel, and tracks all user activities to maintain an audit trail.
By adopting Yuva’s ARS eTMF solution, organizations involved in clinical trials can streamline document management processes, enhance collaboration, improve document quality and completeness, and ensure regulatory compliance. It facilitates efficient access to trial documentation, ultimately supporting the successful execution of clinical research studies.

